Hunniwell Lake Ventures || Resources
COVID-19 Open Grant List

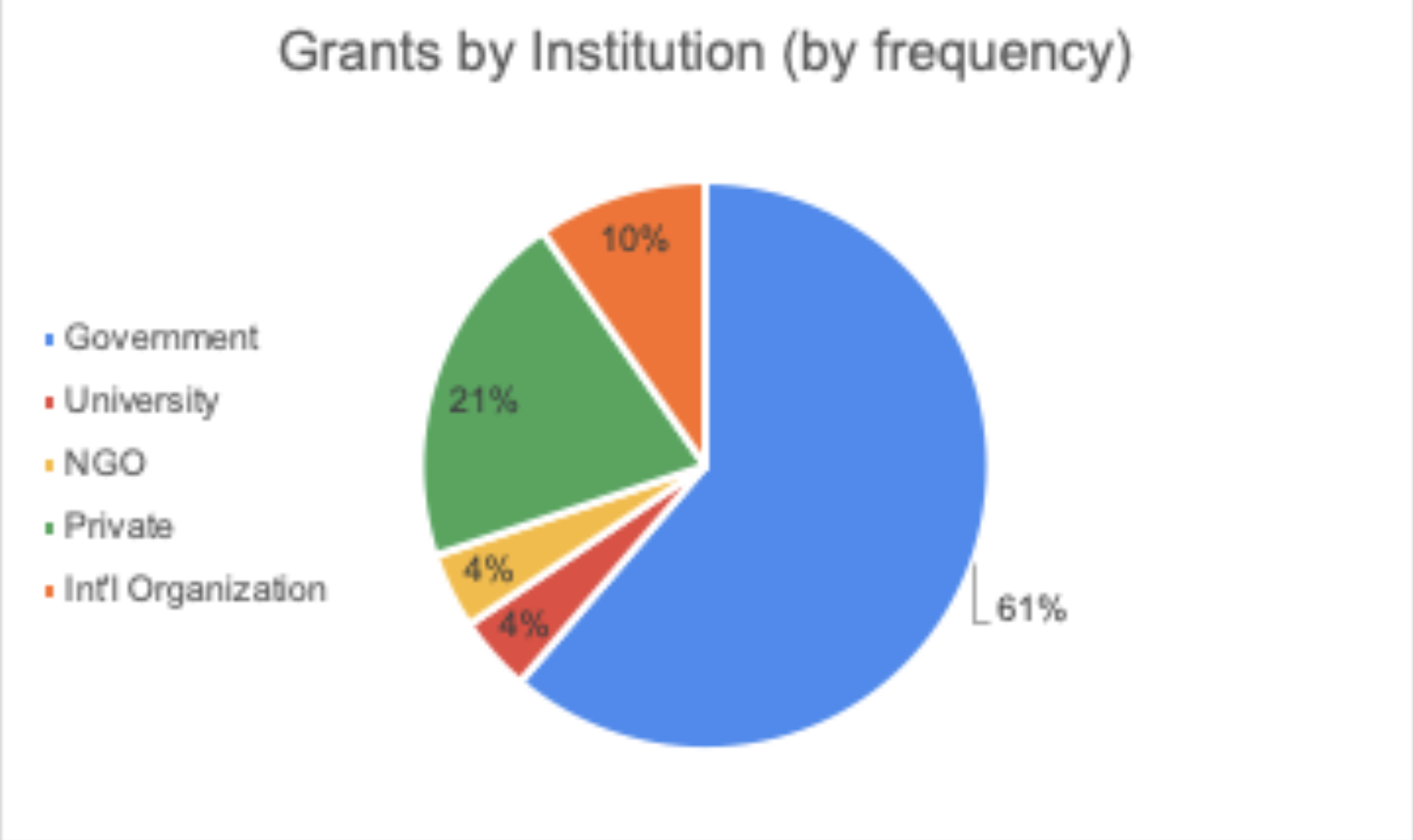
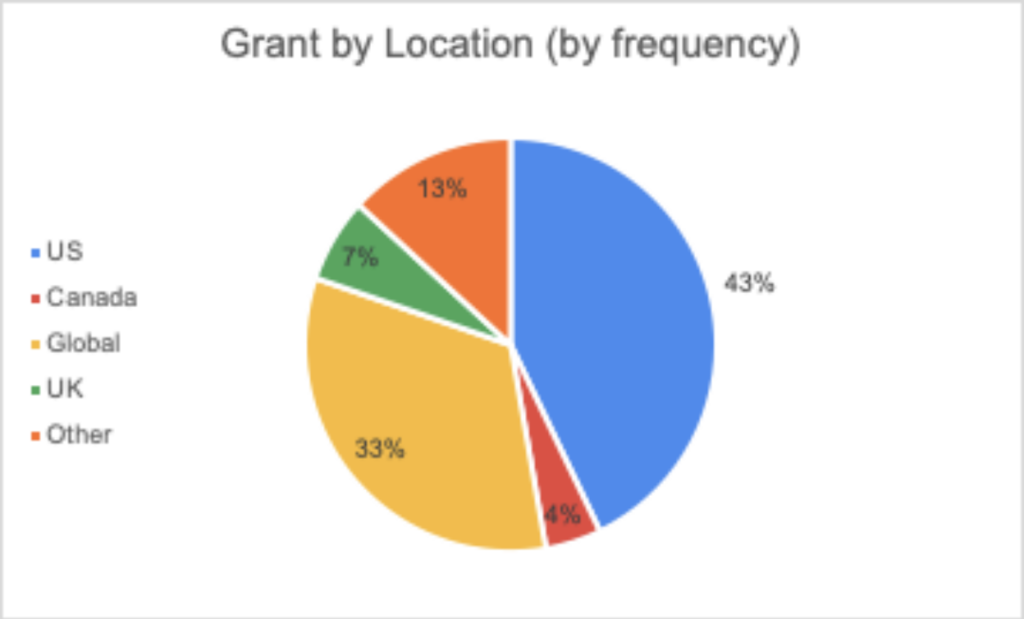
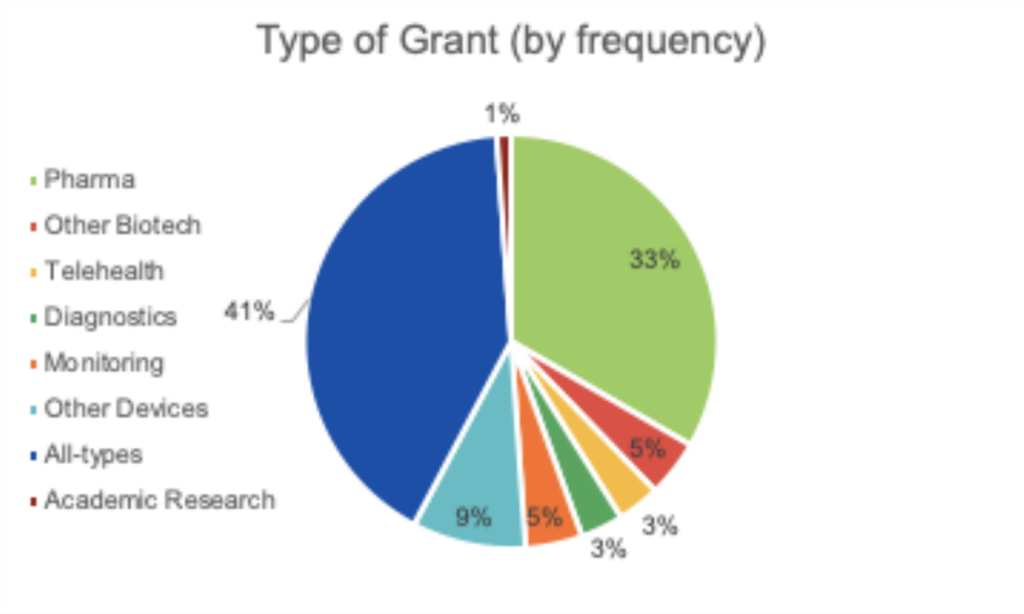
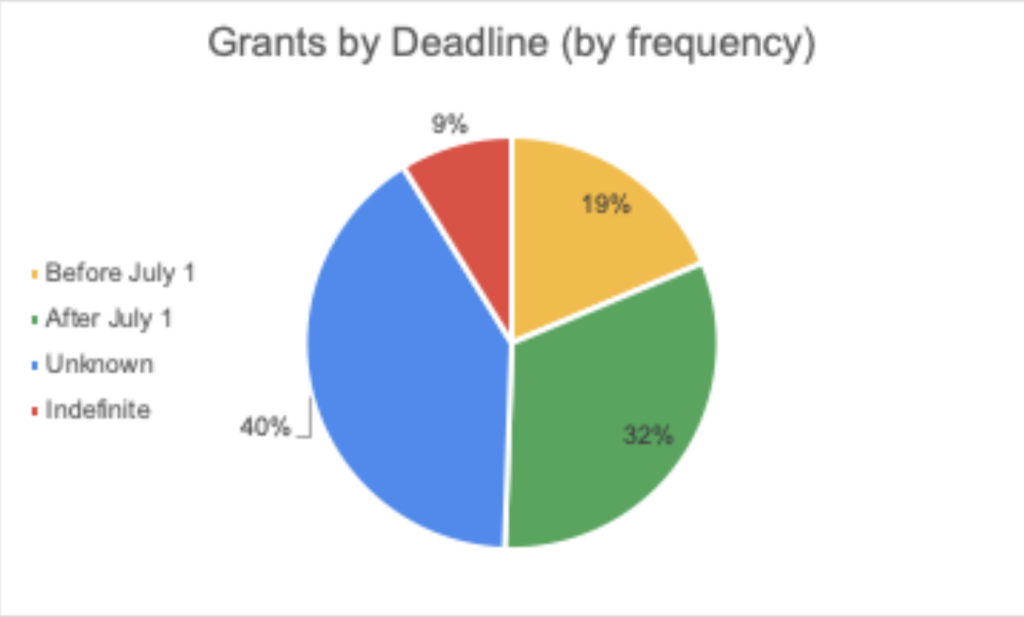
June 16, 2020 || With the world singularly united in efforts to solve the challenges presented by COVID-19, there is an international call for research and innovations to fight the pandemic. Hunniwell Lake Ventures has compiled a list of active COVID-19 grants, globally sourced from governments and organizations, seeking a variety of solutions and reliefs. This list is sorted by deadline (before July 1, after July 1, Indefinite, and Unknown), notes if geographic restrictions have been placed on a grant. A general categorization of the grant’s request, as well as their available funding are provided as well. We encourage readers to bring additional grants to our attention and correct any present errors by emailing us at comms@hunniwell.com. Picture courtesy of Shutterstock.
Please click the grant name to reach its website for more information. This is an ongoing list of grants focused on supporting COVID-19 efforts and will be continually updated. Please reach out to comms@hunniwell.com to contribute to this list or would like more information.
| Name of Grant | Type of Activity Supported | Grantor | Grantor Classification | Deadline | Total funding available | Applicant Geographic Restrictions | Estimated funding per grant |
|---|---|---|---|---|---|---|---|
| Bench Testing Therapeutic/Indication Pairing Strategies | Pharma | National Center for Advancing Translational PAR-17-465 Sciences/NIH/DHHS | Government | 2020-06-26 | Not Available | US (foreign components allowed, foreign institutions are not) | 700000 |
| RFA-AI-20-028 -- Partnerships for Countermeasures against Select Pathogens (R01 Clinical Trials Not Allowed) | Pharma | National Institute of Allergy and Infectious Diseases/NIH/DHHS | Government | 2020-06-29 | 10500000 | US | $750,000 a year for up to 5 years |
| COVID-19 Challenges | Other Biotech | Department of National Defence (DND) | Government | 2020-06-23 | 11116792.95 | Canada | $200,000 for Phase I, $1,000,000 for Phase II (CAD) |
| NIBIB Trailblazer Award for New and Early Stage Investigators (R21 Clinical Trial Optional) | Other Biotech | National Institute of Biomedical Imaging and Bioengineering (NIBIB) | Government | 2020-06-16 | Not Available | US | $400000 over 3 years |
| NIBIB Exploratory/Developmental Research Grant Program (R21 Clinical Trial Optional) | All-types | National Institute of Biomedical Imaging and Bioengineering (NIBIB) | Government | 2020-06-16 | Not Available | $275,000 over 2 years | |
| NIH Small Research Grant Program (Parent R03 Clinical Trial Not Allowed) | All-types | National Institute of Biomedical Imaging and Bioengineering (NIBIB) | Government | 2020-06-16 | Not Available | 50000 | |
| Achieving an unprecedented acceleration of vaccine development and global manufacturing capacity to prevent COVID-19 | Pharma | Coalition for Epidemic Preparedness Innovations (CEPI) | Int'l Organization | 2020-06-30 | Not Available | Switzerland | Covers expenses |
| The COVID-19 Detect & Protect Challenge | All-types | The United Nations Development Programs Centre for Technology, Innovation and Sustainable Development, Hackster.io | Int'l Organization | 2020-06-30 | 25000 | $500 or $1500 depending on the award | |
| AWS Diagnostic Development Initiative (DDI) | Diagnostics | Amazon Web Services | Private | 2020-06-20 | 20000000 | In-kind | |
| Special call for proposals: Fight SARS-CoV-2 | All-types | DIM ELICIT | Private | 2020-06-30 | €10,000,000 budget | Ile de France region | €15,000 for running/staff and/or €30,000 for equipment totalling €45,000 |
| ATS/GSK Research Grant in COVID-19 | Academic Research | American Thoracic Society (ATS), GlaxoSmithKline | Private | 2020-06-23 | 100000 | 50000 | |
| Scientist Development Award | All-types | Rheumatology Research Foundation | Private | 2020-06-01 for Letter of Intent 2020-07-01 for Application | Not Available | 225000 | |
| Investigator Award | All-types | Rheumatology Research Foundation | Private | 2020-06-01 for Letter of Intent 2020-07-01 for Application | Not Available | 375000 | |
| Innovative Research Award | All-types | Rheumatology Research Foundation | Private | 2020-06-01 for Letter of Intent 2020-07-01 for Application | Not Available | 400000 | |
| Innovative Research Award for Community Practitioners | All-types | Rheumatology Research Foundation | Private | 2020-06-01 for Letter of Intent 2020-08-03 for Application | Not Available | 400000 | |
| Elevate Prize | All-types | Elevate Prize Foundation, MIT Solve | University | 2020-06-29 | 5000000 | 300000 | |
| Health Security and Pandemics Challenge | All-types | MIT Solve | University | 2020-06-18 | Not Available | US | 10000 |
| PHS 2019-02 Omnibus Solicitation of the NIH, CDC, and FDA for Small Business Innovation Research Grant Applications (Parent SBIR [R43/R44] Clinical Trial Not Allowed) | All-types | National Institute of Biomedical Imaging and Bioengineering (NIBIB) | Government | 2020-07-07 | Not Available | US | $252,131 for Phase I $1,680,879 for Phase II |
| PHS 2019-02 Omnibus Solicitation of the NIH for Small Business Technology Transfer Grant Applications (Parent STTR [R41/R42] Clinical Trial Not Allowed | All-types | National Institute of Biomedical Imaging and Bioengineering (NIBIB) | Government | 2020-07-07 | Not Available | US | $252,131 for Phase I $1,680,879 for Phase II |
| Medical Robotics for Contagious Diseases Challenge | Other Biotech | EPSRC UK-Robotics Autonomous Systems (UK-RAS) Network | Government | 2020-09-30 | Not Available | UK | £15,000 |
| Biomedical Advanced Research and Development Authority (BARDA) Broad Agency Announcement (BAA) | Pharma | Department of Health & Human Services | Government | 2020-10-31 | Not Available | US | Not available |
| RFP--AMENDMENT--Special Notice for Innovative Commercial Products in Support of CORONAVIRUS (COVID-19) Response | Diagnostics | Department of Homeland Security | Government | 2020-08-31 | Not Available | US | Not available, not guaranteed |
| Ecology and Evolution of Infectious Diseases (EEID) | Monitoring | National Science Foundation | Government | 2020-11-18 | 24000000 | US | 2400000 |
| RFP - CSO COVID-19 Response | All-types | Department of the Air Force | Government | 2020-09-30 | Not Available | US | Not available |
| Prototype Development to Combat COVID-19 | All-types | DOD - Department of the Army, Medical Technology Enterprise Consortium | Government | 2020 - Projected mid-2020 | 20000000 | US | 4000000 |
| Dear Colleague Letter: Request for SBIR/STTR Phase I Proposals Addressing COVID-19 (NSF 20-065) | All-types | National Science Foundation | Government | 2020-09-03, 2020-09-04, 2020-12-03 | 2500000000 | US | $256,000 until Phase I award, then up to $1,500,000 additional funding |
| Biomedical Advanced Research and Development Authority (BARDA) Broad Agency Announcement (BAA) | All-types | Department of Health & Human Services | Government | 2020-10-31 | Not Specified | US | Not available |
| Collaborative Cross (CC) Mouse Model Generation and Discovery of Immunoregulatory Mechanisms (R21 Clinical Trial Not Allowed) | Pharma | National Institute of Allergy and Infectious Diseases/NIH/DHHS | Government | 2020-09-03 | 1000000 | US | The combined budget for direct costs for the two-year project period may not exceed $275,000. No more than $200,000 may be requested in any single year. |
| COVID-19 Regional Genomics Initiative | All-types | GenomeCanada | Government | 2022-06-30 | 1111679.29 | Canada | $250,000 (CAD) |
| Call for Multidisciplinary Research into Epidemics and Pandemics | All-types | Deutsche Forschungsgemeinschaft (DFG) | Government | 2020-07-01 for Letters of Intent, 2020-09-01 for proposals | Not Available | Germany | Covers expenses |
| Urgent Funding for Research into Humanitarian Crises Like Epidemics and Pandemics | All-types | Austrian Science Fund (FWF) | Government | 2020-09-30 | 32884550 | Austria | Covers expenses (on average about €275,000) |
| Call for projects RA-COVID-19 | Monitoring | Agence Nationale de la Recherche (ANR) | Government | 2020-10-28 | 17009250 | France | 150000 |
| Global Effort on COVID-19 Health Research (GECO) | Monitoring | National Institute for Health Research (NIHR), UK | Government | 2020-09-28 | Not Available | UK and low and middle-income countries(LMIC) | £1,000,000 |
| COVID-19 Rapid Response Rolling Call | All-types | Medical Research Council UK, UKRI | Government | 2021-04-01 | No limit set | UK | Covers expenses |
| COVID-19 R&D Fund | Pharma | The Malta Council for Science and Technology | Government | 2020-12-31 | 6009935 | Malta | Covers expenses |
| NIH Director’s Emergency Transformative Research Awards (R01 Clinical Trial Optional) | All-types | NIH | Government | 2020-09-30 | 30000000 | US | Covers expenses |
| NIH Director’s Emergency Early Independence Awards (DP5 Clinical Trial Optional) | All-types | NIH | Government | 2030-09-04 | 30000000 | US | 250000 |
| Notice of Intent to Publish a Funding Opportunity Announcement for Serological Sciences Centers of Excellence | Pharma | National Cancer Institute (NCI) | Government | 2020-07-20 | 19000000 | US | either $1,500,000 or $500,000 depending on the type |
| Notice of Special Interest (NOSI): Infrastructure Access for Research on Coronavirus Disease 2019 (COVID-19) Conducted in the National Dental Practice-Based Research Network | All-types | National Institute of Dental and Craniofacial Research (NIDCR) | Government | 2020-11-02 | None | US | In-kind |
| NIBIB Exploratory Clinical Trials for Small Business (R44 Clinical Trial Required) | All-types | National Institute of Biomedical Imaging and Bioengineering (NIBIB) | Government | 2020-09-05, 2021-01-05 | Not Available | US | $225,000 for Phase I $1,500,000 for Phase I |
| Limited Competition: Competitive Revision Awards for the Clinical and Translational Science Award (CTSA) Program (U54 Clinical Trial Optional) | Other Devices | National Center for Advancing Translational Sciences (NCATS) | Government | 2020-07-10, 2020-11-09, 2021-03-08 2021-07-09 | Not Available | US | $750,000 a year |
| Limited Competition: Clinical and Translational Science Award (CTSA) Program: Exploratory Collaborative Innovation Awards (R21 Clinical Trial Optional) | Other Devices | National Center for Advancing Translational Sciences (NCATS) | Government | 2020-07-10, 2020-11-09, 2021-03-08 2021-07-09 | Not Available | US | $275,000 over 2 years |
| Limited Competition: Clinical and Translational Science Award (CTSA) Program: Collaborative Innovation Award (U01 Clinical Trial Optional) | Other Devices | National Center for Advancing Translational Sciences (NCATS) | Government | 2020-09-25 | 9000000 | US | For clinical projects, no more than $750,000 direct costs annual budget should be requested. For non-clinical projects, no more than $400,000 direct costs annual budget should be requested. |
| Fondo Supera COVID-19 | All-types | Banco Santander and Spanish National Research Council (CSIC), Spain | Government, Private and University | 2020-12-18 | 5669750 | Spain | Covers expenses |
| AMable Open Call for Solution Ideas (COVID-19) | All-types | AMable | Int'l Organization | 2020-10-01 | 396882.5 | Europe | €10000 per challenge set, up to €60,000 |
| Research Grant for Pandemic Preparedness | All-types | Merck KGaA | Private | 2020-08-31 | Not Available | 1500000 | |
| Emergency Awards: Rapid Investigation of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) and Coronavirus Disease 2019 (COVID-19) (R21 Clinical Trial Not Allowed) | Pharma | Department of Health & Human Services | Government | Rolling Submission | Not available | US | 275000 |
| RFP -- AMENDMENT --- BARDA's Division of Research, Innovation & Ventures (DRIVe) Easy Broad Agency Announcement | All-types | Department of Health & Human Services | Government | Continuous | 750000 | US | Not available |
| Dear Colleague Letter--Coronavirus Disease 2019 (COVID-19) | Pharma | Department of Energy | Government | Continuous Submission | Not Specified | US | In-kind |
| Pharma | FAPERJ and the State of Rio de Janeiro, Brazil | Government | Continuous | $30,000,000 (Brazilian Reals) | Brazil | Covers expenses | |
| Special Call for COVID-19 Projects | Pharma | California's Institute for Regenerative Medicine (CIRM) | Government | 2020-05-07, new deadline every 2 weeks following | 5000000 | Clinical trial $750,000 Late stage preclinical $400,000 Translational $350,000 Discovery $150,000 |
|
| Fast-Track Program for COVID-19 Test Development and Distribution Innovative Technologies to Increase U.S. Capacity for COVID-19 Testing | Diagnostics | National Institute of Biomedical Imaging and Bioengineering (NIBIB) | Government | Rolling Basis | 500000000 | Phase 0: $25,000 Phase 1: $500,000 - < $10,000,000 Phase 2: $10,000,00 + |
|
| SARS-COV-2 Diagnostics: Performance Data | Other Devices | Foundation for Innovative New Diagnostics (FIND) | NGO | Rolling Basis | Not Available | Not available | |
| Emergency COVID-19 Research Seed Funding | All-types | University of California | University | Continuous until funding expires | 2000000 | California | 25000 |
| Urgent Competitive Revision to Existing NIH Grants and Cooperative Agreements (Urgent Supplement - Clinical Trial Optional) | Pharma | National Institutes of Health/DHSS | Government | None Posted | Not Available | US | Not available |
| Expedited, Rapid Access Call for COVID-19 Research | Pharma | Argonne National Laboratory | Government | None Posted | Not Available | US | In-kind |
| COVID-19 Research Support | Pharma | Brookhaven National Laboratory | Government | None Posted | Not Available | US | In-kind |
| Notice of Information: Contributing to the Global COVD-19 Crisis response by Allowing Some NCI-supported Projects to be Redirected to COVID-19-related Research During the Crisis | Pharma | National Institutes of Health/DHSS | Government | None Posted | Not Available | US | Not available |
| Rapid Access for COVID-19 Research | Pharma | Oak Ridge National Laboratory | Government | None Posted | Not Available | In-kind | |
| COVID-19 Telehealth Program | Telehealth | Federal Communications Commission | Government | None Posted | 200000000 | US | 1000000 |
| Priority Access Call for Work on Combating COVID-19 | Pharma | Paul Scherrer Institut (PSI) / Swiss Light Source (SLS) / SwissFEL | Government | None Posted | Not Available | In-kind | |
| Emergency Competitive Revision to Existing NIH Awards (Emergency Supplement - Clinical Trial Optional) | All-types | National Institutes of Health (multiple ICs) | Government | Varies by IC | Application budgets not limited | Not available | |
| Urgent Competitive Revision to Existing NIH Grants and Cooperative Agreements (Urgent Supplement - Clinical Trial Optional) | Pharma | National Institutes of Health/DHHS | Government | Varies by IC | Not Available | US | Application budgets not limited |
| Administrative Supplements to Existing NIH Grants and Cooperative Agreements (Parent Admin Supp Clinical Trial Optional) | Pharma | National Institutes of Health/DHHS | Government | Varies by IC | Not Available | US | Application budgets are limited to no more than the amount of the current parent award, and must reflect the actual needs of the proposed project. |
| SOLICITUD URGENTE DE EXPRESIONES DE INTERÉS PARA LA FINANCIACIÓN EXTRAORDINARIA DE PROYECTOS DE INVESTIGACIÓN SOBRE EL SARS-COV-2 Y LA ENFERMEDAD COVID-19 | All-types | Spanish Instituto de Salud Carlos III (ISCIII) | Government | None Posted | 27214800 | Spain | |
| COVID-19 Rapid Response | All-types | Science Foundation Ireland, Enterprise Ireland, IDA Ireland | Government | None Posted | Ireland | Covers expenses | |
| COVID-19 HPC Consortium | All-types | White House Office of Science and Technology Policy, the U.S. Department of Energy and IBM | Government | None Posted | Not Available | In-kind | |
| Research and innovation ideas to address Covid-19 | Telehealth | Arts and Humanities Research Council, UK / UKRI | Government | None Posted | Not Available | Covers 80% of expenses | |
| Rapid Access Call for Octopus Facility Proposals: SARS-CoV-2 | Pharma | Central Laser Facility, Science and Technology Facilities Council | Government | None Posted | Not Available | UK | In-kind |
| NRC Pandemic Response Challenge Program | Pharma, Diagnosis or Telehealth | National Research Council of Canada (NRC) | Government | None Posted | 11116792.95 | Canada | Covers expenses |
| Biomanufacturing capacity at Royalmount: NRC Human Health Therapeutics Research Centre | Pharma | Human Healths Therapeutic Research Centre | Government | None Posted | 11116792.95 | Canada | In-kind |
| 2020 COVID-19 FlexGrants | Other Biotech | West Coast Consortium for Technology & Innovation in Pediatrics | Government | None Posted | Not Available | US | 15000 |
| ICESCO Prize for Fighting Against Coronavirus | Pharma | Islamic World Education, Scientific, and Cultural Organization | Int'l Organization | None Posted | 200000 | Morocco | 200000 |
| Deuteration & Macromolecular Crystallisation (DEMAX) COVID-19 related Research Projects | Pharma | European Spallation Source | Int'l Organization | None Posted | Not Available | In-kind | |
| Fast Track Call for Requests for Computing Resources | All-types | Partnership for Advanced Computing in Europe (PRACE) | Int'l Organization | None Posted | Europe | In-kind | |
| COVID-19 Fast Track Access | Pharma | Central European Research Infrastructure Consortium (CERIC) | Int'l Organization | None Posted | In-kind | ||
| ICESCO Prize for Fighting Novel Coronavirus (Covid-19) | Pharma | Islamic World Educational, Scientific and Cultural Organization (ICESCO) | Int'l Organization | None Posted | 200000 | 200000 | |
| Covid-19 scientific research | All-types | European Synchrotron Radiation Facility (ESRF) | Int'l Organization | None Posted | Not Available | In-kind | |
| Competitive Fund for Peace and Recovery | Monitoring | Innovations for Poverty Action (IPA) | NGO | See website for deadlines and how to apply | Not Available | No high income countries, focus on UK Department for International Development (DFID) priority countries | 50000 |
| Get funding for ideas that address COVID-19 | All-types | UK Research and Innovation (UKRI) | NGO | None Posted | No limit set | UK | Covers 80% of expenses |
| COVID-19 Young Leaders Fund | All-types | One Young World | NGO | Not Available | Not Available | 6000 | |
| Access to Platform for Partner COVID-19 Projects | All-types | Ginkgo Bioworks | Private | None Posted | 25000000 | US | In-kind |
| COVID-19 Vaccine Development Award | Pharma | MMS Holdings Inc. | Private | None Posted | 1000000 | In-kind | |
| Ferring COVID-19 Investigational Grants | Monitoring | Ferring Pharmaceuticals | Private | None Posted | Not Available | 25000 | |
| PCORI Funding Opportunity for COVID-19-Related Enhancements to Existing PCORI-Funded Dissemination and Implementation Awards | Other Devices | Patient-Centered Outcomes Research Institute (PCORI) | Private | None Posted | Not Available | US, foreign companies that demonstrate benefit to US healthcare/citizens | 500000 |
| PCORI Funding Opportunity for COVID-19-Related Enhancements to Existing PCORI-Funded Research Projects | Other Devices | Patient-Centered Outcomes Research Institute (PCORI) | Private | None Posted | Not Available | US, foreign companies that demonstrate benefit to US healthcare/citizens | 500000 |
| PCORI Funding Opportunity for COVID-19-Related Enhancements to Existing PCORI-Funded Engagement Awards | Other Devices | Patient-Centered Outcomes Research Institute (PCORI) | Private | None Posted | Not Available | US, foreign companies that demonstrate benefit to US healthcare/citizens | 150000 |
| The COVID-19 Therapeutics Accelerator | Pharma | Wellcome, Bill & Melinda Gates Foundation and Mastercard | Private | None Posted | 125000000 | Three posted examples all have $20,000,000 grants | |
| Novartis COVID-19 Response Fund | Other Devices | Novartis | Private | None Posted | 20000000 | 1000000 | |
| Labguru Pro-bono COVID-19 Programme | Pharma | BioData | Private | None Posted | 1000000 | In-kind | |
| Pharma | COVID-19 Early Treatment Fund (CETF) | Private | None Posted | 30000000 | <$1,000,000, past grants awarded average $300,000 |
![]()
Hunniwell Lake Ventures (HLV) is a Palo Alto-based VC firm that invests exclusively in medical devices. Its mission is to make surgery safer, more accessible and less invasive by investing in innovations that help surgeons visualize the operative field better, employ surgical tools using improved and advanced technologies to treat the patient, and ensure their speedy recovery through the best wound closure and tissue healing technologies available.