Hunniwell Lake Ventures || Spotlight Series
The Mechanical Destruction of COVID
July 22, 2020 || The newly launched company, Shape Theranostics presents an ideal solution to mass disease prevention. CEO Irving Weinberg discusses the power of his magnetic microdevices for targeted mechanical destruction of coronaviruses and other diseases.

Read more on Hunniwell Lake Ventures COVID-19 Resources
Irving Weinberg is a practicing radiologist and the president of Weinberg Medical Physics. After studying medical physics at UCLA, he received a medical degree from the University of Miami and completed his radiology and nuclear medicine residency at Johns Hopkins University. He later founded and was the Chief Scientific Officer of Naviscan PET Systems, Inc., a company that built the first PET scanner for breast imaging. In 2008, he launched Weinberg Medical Physics as a medical device incubator and helped develop novel medical imaging and interventional technologies into successful companies. Over his career, his FDA-approved medical devices have been used by over one million Americans
Sahar Jafari is the president of Shape Theranostics. She earned her PhD in material science and engineering from a joint program between her home country of Iran, Toyota, and the National Institute for Material Science in Japan. During both her masters and PhD, she worked with magnetic materials, which led to her meeting Weinberg at George Mason University.
More recently, Weinberg and Jafari have launched Shape Theranostics, a medical device start-up company that is developing magnetic microdevices for targeted mechanical destruction of coronaviruses and other shape-defined pathogens. With the emergence of the COVID-19 pandemic, this device has the potential to protect millions of people who are exposed to the virus.
- Tell us about yourself and your background.
- How do the magnets work?
- Explain your device’s testing process. Are you able to fast track the testing process? Could you share the results or progress you have made?
- Who are your intended customers for your device?
- Have you had any changing relationships with regulatory agencies because of COVID-19?
- How do you think COVID-19 may impact your industry?
- What’s next for your company?
Tell us about yourself and your background.
Irving Weinberg: After my medical training, I spent about two years working at the NIH for my first device, a PET scanner for the breast, before I started launching companies. I am still practicing radiology, but my focus is to run this incubator for about five days a week and spin-off medical imaging or image-guided therapy companies by matching a president with an experienced and knowledgeable CEO. We’ve had great success and have spun off about five companies in the past four years.
Sahar Jafari: Although I focused on non-medical material sciences for my Ph.D., I worked on synthesizing magnetic materials for cancer treatments for my Masters, which inspired me to go back to bioengineering to help patients. In 2016, I met Irving at George Mason University, and I started working on projects making magnetic particles for different bio applications with him.
How do the magnets work?
Irving Weinberg: By applying an external magnetic field from outside of the body, we can guide the magnets to the exact tissue or desired destination. Then, we turn off the magnetic field to stabilize the magnets in their place and relax the particles. Because the distance involved is so small, about 100 nanometers to be exact, the force between the magnets is stronger than the atmosphere, which is enough to break the capsid of the virus. As the particles are opening and closing, viruses can get trapped between the two anvils. We’ve done some experiments with inactivated viruses and have had promising results.
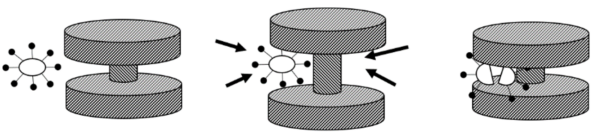
Micron-sized magnetic anvils separated by compressible spacers, the device opens and closes under the influence of the externally-applied magnetic field, drawing in nearby pathogens like a jellyfish and cracking them. The gap between the anvils can be tailored to destroy specific shape-defined pathogens, such as a COVID-19 capsid, without harming smaller or larger native structures. After the capsid is cracked, the RNA is deactivated by the device coating or native enzymes. (Image from Shape Theranostics).
Explain your device’s testing process. Are you able to fast track the testing process? Could you share the results or progress you have made?
We are a theranostic - not only will it be able to kill the virus, but we’ll be able to measure a
patient’s viral load... A theranostic could identify that someone has thousands of virus capsids.
— Irving Weinberg, CEO of Shape Theranostics
Irving Weinberg: Our initial niche is people who have been exposed to COVID-19, but have not contracted the illness yet. While a vaccine accomplishes something similar, strength and reliability are variable, which we can see from the inefficacy of HIV, common cold, and flu vaccinations. Moreover, while we can treat everyone, the ideal situation is mass prevention.
This is where our device presents a solution. We are a theranostic — not only will it be able to kill the virus, but we’ll be able to measure a patient’s viral load. If they do not have a fever or present any symptoms, it’s difficult to diagnose them accurately. A theranostic could identify that someone has thousands of virus capsids, meaning they are likely to get the disease and should be pre-treated.
Who are your intended customers for your device?
Irving Weinberg: Our initial niche is people who have been exposed to COVID-19, but have not contracted the illness yet. While a vaccine accomplishes something similar, strength and reliability are variable, which we can see from the inefficacy of HIV, common cold, and flu vaccinations. Moreover, while we can treat everyone, the ideal situation is mass prevention.
This is where our device presents a solution. We are a theranostic — not only will it be able to kill the virus, but we’ll be able to measure a patient’s viral load. If they do not have a fever or present any symptoms, it’s difficult to diagnose them accurately. A theranostic could identify that someone has thousands of virus capsids, meaning they are likely to get the disease and should be pre-treated.
Irving hopes to administer the microdevice either intra-nasally or intravenously. After administration, the device inactivate viruses before macrophages arrive, which take approximately 3 days in the upper respiratory tract. If device is injected intravenously, as is currently being done with stem cells, the device could also reach the lungs. (Taken from the Shape Theranostics).
Have you had any changing relationships with regulatory agencies because of COVID-19?
Irving Weinberg: We haven’t submitted an application for this device yet, and are working through more design work and studies before we approach the FDA. However, we are considering an Emergency Use Authorization to help us fast track this device through clinical trials and a 510k submission. We believe that our device will not only be useful to fight the COVID-19 pandemic, but also other viruses and diseases in the future. COVID is creating a dynamic situation, so our goal is to adapt quickly to the ever-changing environment.
How do you think COVID-19 may impact your industry?
Irving Weinberg: We have had a strong and close collaboration with Chinese investors and manufacturers; however, the pandemic has definitely made it difficult to collaborate and changed the investment environment. The uncertainties and barriers that COVID-19 has created has made it difficult to move quickly. Since we’re a small and nimble company, we are able to navigate through these difficult obstacles.
The uncertainties and barriers that COVID-19 has created has made it difficult to move quickly.
Since we’re a small and nimble company, we are able to navigate through these difficult obstacles.
— Irving Weinberg, CEO of Shape Theranostics
What’s next for your company?
Irving Weinberg: The progression of our device is first: we have to optimize the particles for this particular mission and conduct animal trials with mice. We will then apply for clinical trials at the FDA, and we are hopeful that precedent devices will ease our path to regulatory approval. We believe that we can develop a protocol efficiently for our human trials.
![]()
Hunniwell Lake Ventures is featuring a series of healthcare startups in the fight against COVID-19 to honor their work and persistence during this pandemic. As a venture investor specializing in medical devices, Hunniwell Lake Ventures is well-positioned to help our colleagues address the COVID-19 scientific complex, patient care, and public health crisis.
